Local Insights Into Electrochemical Processes
Electrochemically induced ion insertion is the key phenomenon involved not only in different electrochemical energy storage devices including batteries, electrochemical double-layer capacitors, and pseudocapacitors, but also in electrochemical actuators, neuromorphic computing, and electrochemical water desalination. The ion-hosting electrode most often undergoes significant volume changes driven by Coulomb interactions, bond length changes, or phase changes which is described as electro-chemo-mechanical coupling. Understanding the mechanical response of an electrode during electrochemical cycling and its correlation to the device’s electrochemical performance and stored charge is crucial to improving the performance of insertion-type energy storage devices, electrochemical actuators, water purification, ion separation, and neuromorphic computing applications.
AFMs can be used to track local volume changes in-situ and can, therefore, can be used to study local electrochemical processes on the level of single grains or electrode components which is not possible with current-based characterization techniques. One of the goals of this research direction is to build a library of strain-charge coupling phenomena for different electrochemical energy storage devices and explore the consequences of local heterogeneity of electro-chemo-mechanical on electrochemical performances and local ionic transport.
Specifically, electrode volume changes and stiffness changes as a result of (a) electric double layer formation, as well as (b) surface and (c) bulk redox processes, are investigated. Of high interest are layered electrode materials since they allow for fast ionic transport, have an interlayer space that can be modified, and typically accommodate strain without mechanical degradation such as crack formation. Using an AFM, local current-strain coupling coefficients can be identified and correlated with information about individual grain orientation and can be directly compared to in-situ X-ray studies which track the volume changes on the unit cell level.
Highlights
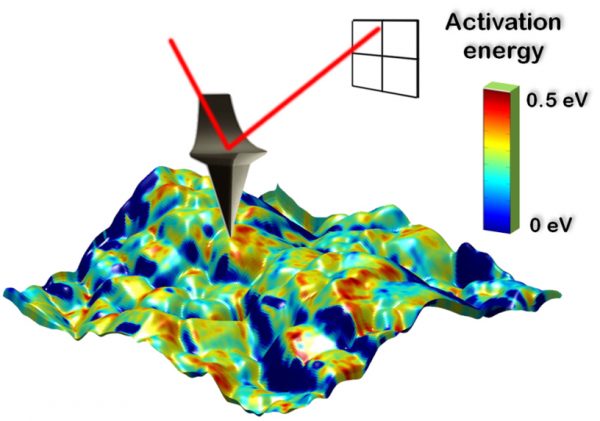 |
Spatially resolved activation energy map overlaid with sample topography for Li-ion transport in LiCoO2 thin film cathode material. The average activation energy can be extracted to 0.26 eV, which fits well with macroscopic measurements and theoretical calculations (blue areas indicate not enough signal to be measured). |
 |
The electro-chemo-mechanical coupling behaviors of proton insertion into WO3-based electrodes and the charging heterogeneity was revealed by mechanical CV (mCV) approach via operando AFM.
“Probing local electrochemistry via mechanical cyclic voltammetry curves” Nano Energy 81, (2021) |
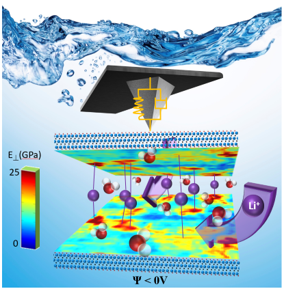 |
Large and tunable elastic properties of two dimensional MXene (Ti3C2) during charging/discharging have been quantified at different Li-ion contents in-situ in a liquid environment allowing to identify ion insertion pathways.
“Nanoscale Elastic Changes in 2D Ti3C2Tx Pseudocapacitive Electrodes” Adv. Energy Mat., (2016) |